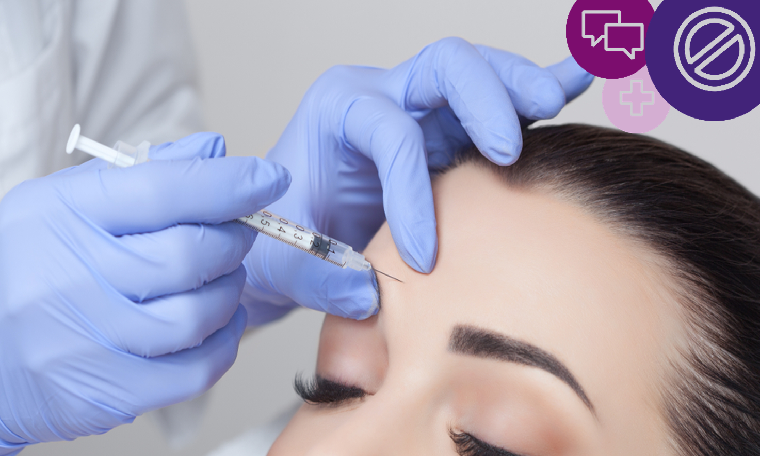
Botulinum toxin, such as Botox and other brands (including Vistabel, Dysport, Bocouture and Azzalure, etc.) is a prescription only medicine and that means it can’t be advertised to the public in any media. The ASA has investigated several complaints about the direct and indirect promotion of Botox and here are some key dos and don’ts.
Don’t make direct or indirect references to Botox
Remember – the prohibition includes references in images and hashtags like #botox, as well as ads and promotions offering Botox treatment as a competition prize, or as part of a treatment package. Ads in all media are subject to the rules. There’s no getting round them by tweaking the product’s name (beautox is an example), or even leaving it out altogether and referring to anti-wrinkle injections if what you’re offering is Botox.
Indirect references have the same effect as direct references and readers will understand that both promote Botox.
Don’t use medical professionals or celebrities to endorse medicines
Social media ads fell afoul of rule 12.18, when the ASA ruled that the reality TV star in the ad had the attention of a large number of people and was therefore a ‘celebrity’.
Don’t exploit readers’ insecurities
As well as banning it for advertising Botox, the ASA also looked at whether this ad was irresponsible and harmful. It concluded that the ad’s focus on women, particularly mothers, exploited women’s insecurities around aging, and perpetuated the harmful gender stereotype that women should look a certain way. Phrases like “back to school Botox” were especially frowned upon.
Is there anything you can say about Botox in advertising?
Do advertise directly to healthcare professionals in media addressed only to them. They are defined as people “qualified to prescribe or supply” medicines and so are not usually beauty practitioners, unless they also have those qualifications. Ads must not appear in media likely to be seen by the general public.
Do promote consultations, if you are a clinic or pharmacy and Botox is one of several facial treatments you offer. But it must be clear that discussion of various treatment options will take place and a product won’t be sold or administered if a customer isn’t suitable.
Do give balanced and factual information, along the lines of that found in the patient information leaflet, ‘Summary of Product Characteristics’ or similar non-promotional information that comes with the product, on your website. This should not be on the home page because casually browsing consumers must not be able to come across information relating to Botox or other POMs with ease.
This AdviceOnline entry summarises the main points you need to consider and the CAP Copy Advice team are happy to advise you on any specific queries – more details here.
More on
-
Keep up to date
Sign up to our rulings, newsletters and emargoed access for Press. Subscribe now.


